Sickle Cell Community
The Sickle Cell Disease Community, an initiative led by St. Jude Children’s Research Hospital, shares whole genome sequencing data and clinical data with research and clinical communities. Our goal is to promote global collaborative efforts to identify genetic modifiers of sickle cell disease complications, improve clinical management for patients, and develop more effective treatments.
About
The Sickle Cell Disease Study shares rich genomic and clinical data from large cohorts of individuals with sickle cell disease. Data may be easily and securely accessed by academic investigators to foster global collaboration. Access the raw genomics data
While sickle cell disease is caused by a single-gene mutation, genetic modifiers strongly influence outcomes. This initiative, led by St. Jude Children's Research Hospital, aims to promote collaborative efforts to understand the genetic underpinnings of sickle cell disease and develop more effective treatments.
Why genomics for sickle cell?
Each year, an estimated 300,000 babies are born with sickle cell disease, which is caused by mutations affecting the HBB gene. The resulting mutant sickle hemoglobin (HbS) causes a chronic and debilitating disease with a high risk of early mortality and recurrent episodes of acute severe pain, chronic pain, cerebrovascular events and progressive organ damage.
Genetic modifiers are known to play critical roles in influencing disease severity, treatment response, morbidity and mortality among patients with sickle cell disease. However, many of these modifiers are poorly characterized or remain to be identified. Whole genome sequencing (WGS), coupled with longitudinal clinical follow-up of a large cohort, represents a powerful approach to identifying genetic modifiers of sickle cell disease, elucidating their contributions, and using the information to guide patient care and management.
Who we are
Collaboration is essential for advancing progress in understanding and treating sickle cell disease. This initiative is a collaborative effort to analyze data from multiple participating hospitals and medical centers including:
- St. Jude Children's Research Hospital, Memphis, TN
- Baylor College of Medicine, Houston, TX
- Methodist University Hospital, Memphis, TN
- Novant Health Hemby Children's Hospital, Charlotte, NC
- OSF Healthcare Children's Hospital of Illinois/ University of Illinois Collage of Medicine, Peoria, IL
- Our Lady of the Lake Children's Hospital, Baton Rouge, LA
About Participants
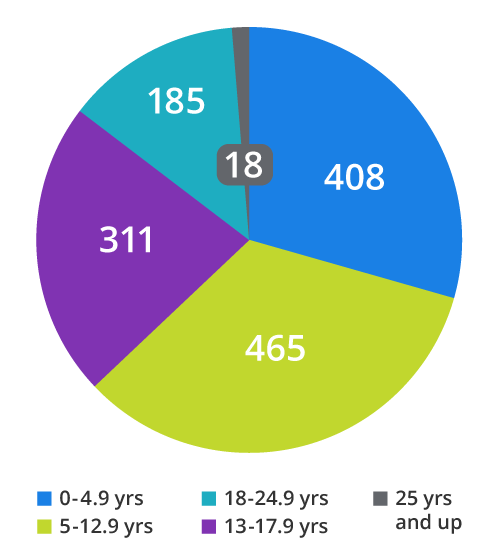
Our major cohort consists of 1,084 participants in the Sickle Cell Clinical Research and Intervention Program (SCCRIP). This longitudinal St. Jude-led observational study is prospectively following individuals with sickle cell disease throughout their lifetimes. We also collect retrospective data to create a record beginning at birth for each participant. View publication. WGS data is available for 503 participants sequenced through SCCRIP (498/503) and other St. Jude studies.
A second major cohort consists of 304 individuals with sickle cell disease who have undergone whole genome sequencing through studies at Baylor College of Medicine in Houston, TX. Genomic DNA was collected from pediatric sickle cell patients receiving care at Texas Children's Sickle Cell Center, with IRB approval. The Center is the second largest pediatric sickle cell center in the United States.
Projects
We are using WGS data coupled with longitudinal clinical follow-up to identify genetic modifiers of co-morbid outcomes for sickle cell disease. Severe pain - a common cause of hospitalization - and early kidney damage are the focus of two initial analyses. The goal is to identify high-risk patients and improve their clinical management.
Pain study
Recurrent acute pain, or vaso-occlusive pain crisis (VOP), is the most common complication of sickle cell disease and correlates strongly with increased hospital visits and early mortality PMID: 1710777. While the genetics of VOP in sickle cell patients has been studied (PMID: 29205277, 27883292, 25102390, 30079801, 22925497, 29531649, 29620434, 19468207, 20172753, 22576309, 30031848, 24136375, 27603703, 29559808), it is not fully understood.
Using WGS, we interrogated the a-thalassemia deletion -a3.7 and 133 candidate risk single nucleotide polymorphisms (SNPs) across an additional 65 genes for association with VOP: 11 SNPs in 3 gene regions associated with fetal hemoglobin (HbF) and 122 additional SNPs in 62 genes previously reported to be associated with pain due to SCD and/or other etiologies. We then constructed unweighted polygenic risk scores (PGSs) by counting the total number of risk alleles per individual across the 11 HbF SNPs (PGSHbF) and the 5 SNPs in COMT (PGSCOMT), where COMT is the gene previously associated with SCD pain (PMID: 29559808, 15537663). We also defined a final PGS comprised of these 16 SNPs plus another 5 internally-validated candidate SNPs (PGSHbF+COMT+5snps), which was more strongly associated with acute VOP than any individual variant. Additionally, patients with the highest 5% of scores had 3-fold more pain events than the bottom 5% but were 5 times more likely to be on hydroxyurea, indicating that patients with high scores might benefit from a second drug.
For a list of SNPs included in each PGS, see the data here.
Renal study
Kidney damage in individuals with sickle cell disease begins in infancy and can progress to kidney failure, which has a high mortality rate. Urinary albumin excretion (albuminuria) is a sensitive marker of early kidney damage in individuals with sickle cell disease. While guidelines from the National Heart, Lung, and Blood Institute (NHLBI) recommend screening for albuminuria beginning at 10 years of age for all sickle cell patients, genetic testing may allow earlier identification of individuals at risk for kidney disease.
Two variants, G1 (rs73885319/rs60910145) and G2 (rs71785313), in the apolipoprotein L-1 (APOL1) gene confer an increased risk of kidney failure to African Americans without sickle cell disease (PMID: 24206458) and of albuminuria and chronic kidney disease (CKD) in individuals with sickle cell disease (PMID: 21910715).
Using our longitudinal WGS cohort of children and young adults with SCD, we examined the association of these variants with the age of onset of albuminuria. We identified that young individuals with a combination of risk alleles of two of the APOL1 variants (G1/G1, G1/G2, or G2/G2) were more likely to develop albuminuria earlier in childhood.
Check out our Kidney Damage Supporting Figure ➜
In a subsequent project, we evaluated a PGS for albuminuria developed in large, trans-ethnic meta-analysis of primarily adult, European, non-SCD individuals (PMID: 31511532) in our pediatric, African American, SCD cohort. We also assessed known SCD chronic kidney disease risk variants (APOL1 G1/G2, a-thalassemia deletion -a3.7, BCL11A rs1427407, and HMOX1 rs743811) that were undetected in the original PGS. We identified three SNPs that associate individually and in a PGS with time to first albuminuria episode, independent of known SCD risk variants.
How to cite this resource
When publishing manuscripts utilizing data or information from this resource, please cite the papers describing the St. Jude Sickle Cell Clinical Research and Intervention Program, and Sickle Genome Project, and this resource and include the URL https://sickle-cell.stjude.cloud.
Hankins JS#, Estepp JH, Hodges JR, Villavicencio MA, Robison LL, Weiss MJ, Kang G, Schreiber JE, Porter JS, Kaste SC, Saving KL, Bryant PC, Deyo JE, Nottage KA, King AA, Brandow AM, Lebensburger JD, Adesina O, Chou ST, Zemel BS, Smeltzer MP, Wang WC, Gurney JG. Sickle Cell Clinical Research and Intervention Program (SCCRIP): A lifespan cohort study for sickle cell disease progression from the pediatric stage into adulthood. Pediatr Blood Cancer. 2018 Sep;65(9):e27228. PMID: 29797644.
Rampersaud E*, Kang G*, Palmer LE, Rashkin SR, Wang S, Bi W, Alberts NM, Anghelescu D, Barton M, Birch K, Boulos N, Brandow AM, Brooke RJ, Chang TC, Chen W, Cheng Y, Ding J, Easton J, Hodges JR, Kanne CK, Levy S, Mulder H, Patel AP, Puri L, Rosencrance C, Rusch M, Sapkota Y, Sioson E, Sharma A, Tang X, Thrasher A, Wang W, Yao Y, Yasui Y, Yergeau D, Hankins JS, Sheehan VA, Downing JR, Estepp JH, Zhang J, DeBaun M, Wu G#, Weiss MJ#. A polygenic score for acute vaso-occlusive pain in pediatric sickle cell disease. Blood Adv. 2021 Jul 27;5(14):2839-2851. PMID: 34283174.
Palmer LE*, Zhou X*, McLeod C*, Rampersaud E, Estepp JH, Tang X, Wang J, Sioson E, Michael JR, Birch K, Hodges JR, Villavicencio M, Rusch M, Newman S, Mulder H, Easton J, Perry K, Downing JR, Hankins JS, Wu G#, Zhang J#, Weiss MJ#. Data Access and Interactive Visualization of Whole Genome Sequence of Sickle Cell Patients within the St. Jude Cloud. Blood 2018 132 (Supplement 1):723. DOI: 10.1182/blood-2018-99-116597
Contact us
We welcome your questions and comments as we continue to develop this community resource. Please contact us at support@stjude.cloud. You may also contact us if you are interested in exploring individual-level data.